Lewis Structure for Bro3- With Formal Charges
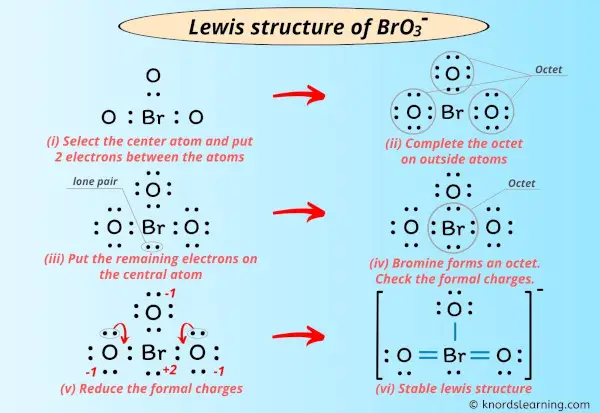
Lewis structure of CH33O. The total valence electron available for drawing the Bromate ion BrO3- lewis structure is 26.

Bro3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
Draw the molecule by placing atoms on the grid and connecting them with bonds.

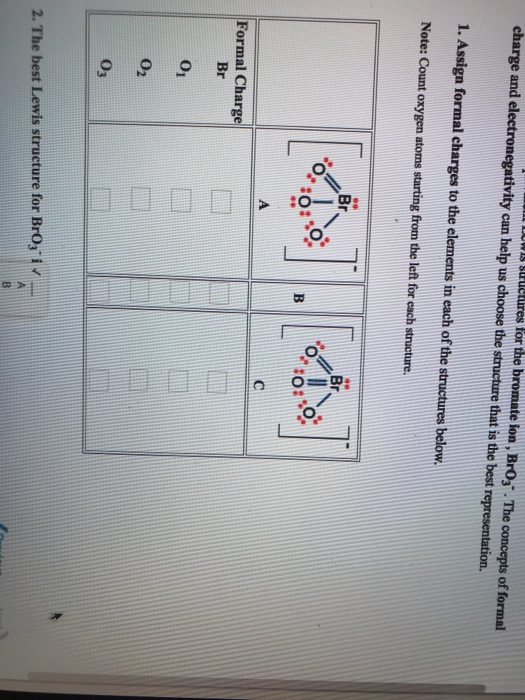
. Write Lewis structures that obey the octet rule for each of the following and assign oxidation numbers and formal charges to each atom. With all single bonds connecting the atoms the formal charge of the O atoms are each -1 while the Br is 2. Starting from this structure complete the correct structure with minimized formal charges.
In the lewis structure of BrO 3 there is one single bond and two double bonds around the bromine atom with three oxygen atoms attached to it. Two bonding pairs and two lone pairs of electrons. For BrO3 B r O 3 Br has 7 valence electrons and O has 6.
The formal charges for atoms in such Lewis structure would be. Or somehow do some of it here and explain. How many TOTAL likely resonance structures exist for BrO3.
The overall formal charge in BrO3- is -1. A 1 B 2 C 3 D 4 E 5 Click to draw a new structure. Include all lone pairs of electrons and formal charges.
BrO3- is a polar molecule because of its the distorted shape that leads to some net dipole moment in it. BrO3- has a total of 26 valence electrons. Use the formula given below- Formal charge valence electrons Non bonding electrons 12 bonding electrons We will calculate the formal charge on the 5th step structure to verify its.
Be sure to show all atoms bonds lone pairs and formal charges. For the following reaction the value of -Δ BrO3-Δt 15 x 10-2 Ms at a particular time. The hybridization in BrO3- is Sp 3.
For the Lewis structure of BrO3- you will have to take formal fees into consideration to search out the most productive Lewis structure for the molecule. This includes the electron represented by the negative charge in BrO 3-. Asked Jun 23 2017 in Chemistry by Angie.
If you could explain it it would help. F C S 6 2 3 1 FCS 6 - 2 3 1 FC S 6 2 3 1 F C O 6 6 1 1 FCO 6 - 61 -1 FC O 6 6 1 1. Write a Lewis structure that obeys the octet rule for BrO3 and assign formal charges to each atom.
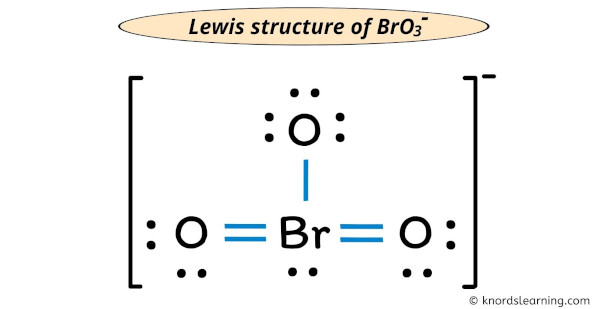
Also note that you should put the BrO3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. There is -1 charge on one oxygen atom in BrO 3-lewis structure. The possible Lewis structure which follows the octet.
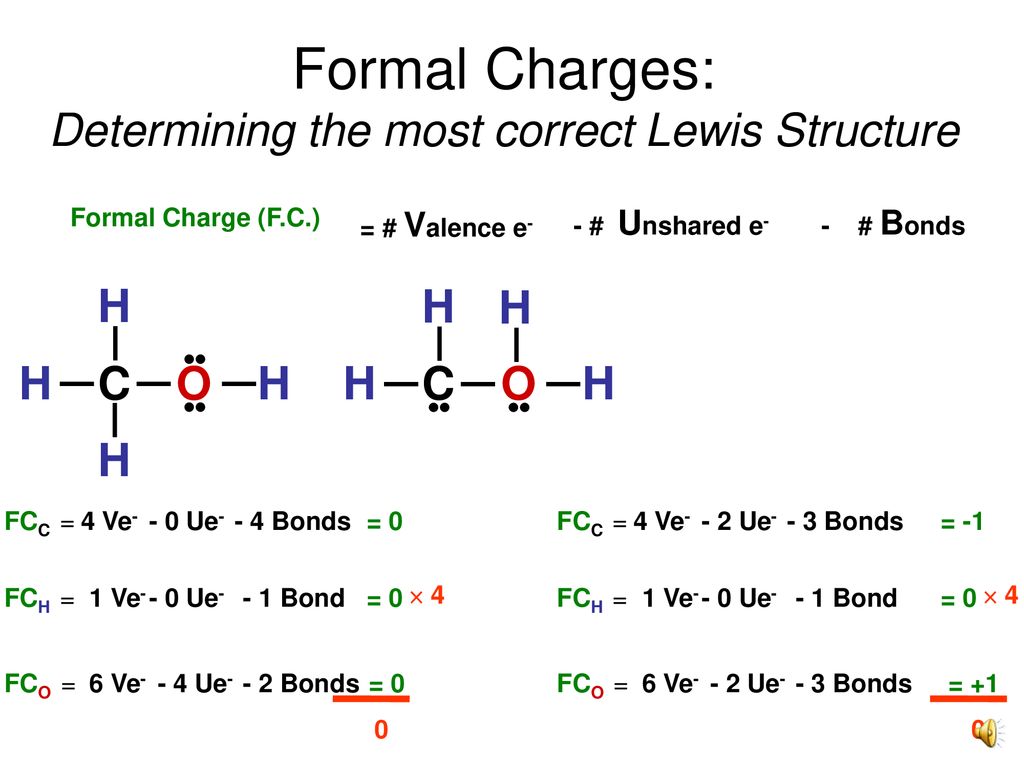
Formal charge is used when creating the Lewis structure of a molecule to determine the charge of a covalent bond. It is helpful if you. Find step-by-step Chemistry solutions and your answer to the following textbook question.
A step-by-step explanation of how to draw the BrO3- Lewis Dot Structure Bromate IonFor the BrO3 - structure use the periodic table to find the total numbe. To do so we first need to draw a Lewis structure for each moleculeion. See full answer below.
Youll want to form double bonds with the central. The oxygen atom with a single bond has three lone pairs the two oxygen atoms with double bonds have two lone pairs and the bromine atom has one lone pair. Lewis structure of BrO 3-contains two BrO bonds and one Br-O bond.
In the Lewis dot formula for the bromate ion BrO3 that minimizes formal charge the central atom is surrounded by asked Jun 23 2017 in Chemistry by Angie a. For the Lewis structure of BrO3- you should take formal charges into account to find the best Lewis structure for the molecule. Oxygen atoms have made bonds with center bromine atom.
From those bonds there are two double bonds and one single bond in the BrO 3-lewis structure. A step-by-step explanation of how to draw the HBrO3 Lewis Dot StructureFor the HBrO3 structure use the periodic table to find the total number of valence el. Convert CH3CHClCHOHCH3 into a skeletal structure.
Also be aware that you simply should put the BrO3- Lewis structure in brackets with as 1- on the outdoor to show that it is an ion with a adverse one price. The bond angle in bromate ion is around 104º. To calculate the formal charge on an atom.
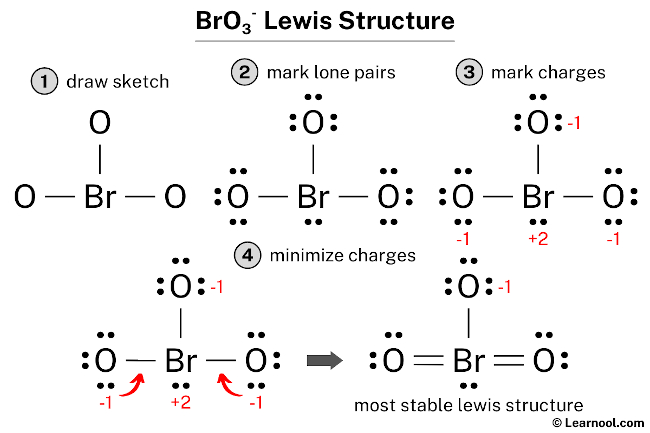
Draw the Lewis structure for the bromate ion BrO3 with minimized formal charges. Also note that you should put the BrO3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. The structure with the formal charge close to zero or zero is the best and most stable lewis structure.
In the Lewis dot formula for the bromate ion BrO3 that minimizes formal charge the central atom is surrounded by. In the Lewis structure Br is placed in the center since it is lower in electronegativity. Im having a lot of trouble with this question.
Formal charge is the difference between the valence electrons unbound valence. Also there is a negative -1 charge on the oxygen atom with a. If you calculate the formal charges for the initial BrO 3-Lewis structure youll find that the Bromine Br has a 1 charge.
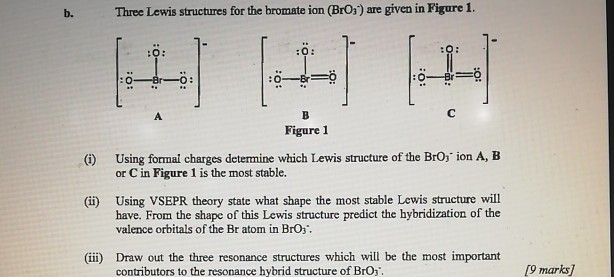
You need to put brackets around the BrO 3-Lewis structure as well as a negative charge to show that the structure is a negative ion. Question 7 of 23 Submit A Lewis structure for bromate BrO3 is shown below however its formal charges are not minimized. The formal charge of an atom can be determined by the following formula.
LatexFC V - N fracB2latex In this formula V represents the number of valence electrons of the atom in isolation N is the number of non-bonding valence electrons and B is the total number of electrons in covalent bonds with other atoms in the molecule. For the Lewis structure of BrO3- you should take formal charges into account to find the best Lewis structure for the molecule.

In The Attached Lewis Structure Of Bro3 Every Atom Bond And Lone Pair Is Positioned To Complete The Structure Label The Atoms With Appropriate Formal Charges Study Com

Formal Charges Determining The Most Correct Lewis Structure Ppt Download

Bro3 Lewis Structure How To Draw The Lewis Structure For Bro3 Youtube
Lewis Structure Of Bro3 With 6 Simple Steps To Draw

Bro3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
Formal Charges Determining The Most Correct Lewis Structure Ppt Download

What Is The Formal Charge On The Bromine Atom In Bro3 Drawn With Three Single Bonds Study Com

Bro3 Lewis Structure Bromate Ion Youtube
Solved Three Lewis Structures For The Bromate Ion Bros Chegg Com
Lewis Structure Of Bro3 With 6 Simple Steps To Draw

Bro3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Geometry Of Molecules Bro3 Lewis Structure Bromate Ion Facebook

Bro3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Bro3 Lewis Structure And Vsepr Geometry Youtube

Bro3 Lewis Structure How To Draw The Lewis Structure For Bro3 Youtube
Solved For The Bromate Ion Bro3 The Concepts Of Formal Chegg Com


Comments
Post a Comment